Details of the Drug
General Information of Drug (ID: DM75U9X)
| Drug Name |
Benzimidazole 5-carboxamide
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
116568-17-3; 1H-Benzo[d]imidazole-6-carboxamide; 1H-benzimidazole-5-carboxamide; 1H-Benzoimidazole-5-carboxylic acid amide; 1H-Benzimidazole-6-carboxamide; 1H-Benzo[d]imidazole-5-carboxamide; 1H-1,3-benzodiazole-5-carboxamide; benzimidazole-5-carboxamide; FNLQDVXHDNFXIY-UHFFFAOYSA-N; ACMC-20mmnb; benzoimidazole-5-carboxamide; SCHEMBL476587; 1H-Benzoimidazole-5-carboxamide; CTK8E3251; CTK0H3052; DTXSID30572624; MolPort-000-353-183; BCP27970; 2711AA; ZINC27986912; STL200279; AKOS002314950; AKOS022171450; FS-2220; MCULE-9328613043
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
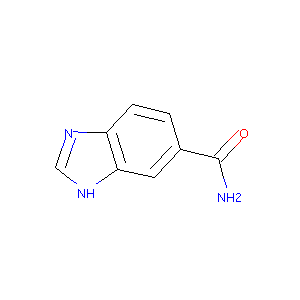 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 161.16 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 0.7 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
