Details of the Drug
General Information of Drug (ID: DM7CNTG)
| Drug Name |
AMG 397
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
UNII-BO0V7196L2; BO0V7196L2; Murizatoclax; Murizatoclax [USAN]; SCHEMBL21040009; WHO 11281; (13S,31R,32R,4R,5E,8S,9R)-6'-Chloro-4-methoxy-8,9-dimethyl-4-(((9aR)-octahydro-2H-pyrido(1,2-a)pyrazin-2-yl)methyl)-3',4'-dihydro-12H,14H,2'Hspiro(10lambda6-thia-11-aza-1(5,7)-(1,5)benzoxazepina-3(1,2)-cyclobutanacyclododecaphan-5-ene-13,1'-naphthalene)-10,10,12-trione; 2245848-05-7; Spiro(5,7-etheno-1H,11H-cyclobut(i)(1,4)oxazepino(3,4-f)(1,2,7)thiadiazacyclohexadecine-2(3H),1'(2'H)-naphthalen)-8(9H)-one, 6'-chloro-3',4',12,13,16,16a,17,18,18a,19-decahydro-16-methoxy-11,12-dimethyl-16-(((9aR)-octahydro-2H-pyrido(1,2-a)pyrazin-2-yl)methyl)-, 10,10-dioxide, (1'S,11R,12S,14E,16R,16aR,18aR)-
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
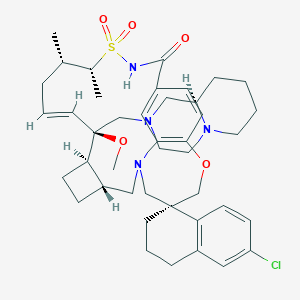 |
||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 2 | Molecular Weight (mw) | 765.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 7.3 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 8 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Refractory hematologic malignancy | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 2A85.5 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
