Details of the Drug
General Information of Drug (ID: DM7EVUF)
| Drug Name |
Asciminib
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
ABL-001; 1492952-76-7; Asciminib free base; ABL001-NX; UNII-L1F3R18W77; L1F3R18W77; NVP-ABL001; (R)-N-(4-(Chlorodifluoromethoxy)phenyl)-6-(3-hydroxypyrrolidin-1-yl)-5-(1H-pyrazol-5-yl)nicotinamide; 1492952-76-7 (free base); N-[4-[chloro(difluoro)methoxy]phenyl]-6-[(3R)-3-hydroxypyrrolidin-1-yl]-5-(1H-pyrazol-5-yl)pyridine-3-carboxamide; Asciminib [USAN]; Asciminib (ABL001); Asciminib (USAN/INN); GTPL8962; CHEMBL4208229; SCHEMBL15388306; TQP0925; EX-A3030; BDBM50459091; NSC789925; s8555; ZINC150275965; CCG-269232; CS-7655; DB12597; NSC-789925; SB18878; BS-15538; HY-104010; D11403; Q27074535; (R)-N-(4-(Chloro difluoromethoxy)phenyl)-6-(3-hydroxypyrrolidin-1-yl)-5-(1H-pyrazol-5-yl)nicotinamide; AY7
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
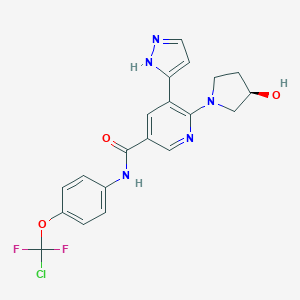 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 449.8 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 6 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 8 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
