Details of the Drug
General Information of Drug (ID: DM7W9MT)
| Drug Name |
KF 17837S
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
152881-18-0; 8-(3,4-Dimethoxyphenyl)-7-methyl-1,3-dipropyl-1H-purine-2,6(3H,7H)-dione; 8-(3,4-dimethoxyphenyl)-7-methyl-1,3-dipropylpurine-2,6-dione; 1,3-Dipropyl-7-methyl-8-(3,4-dimethoxystyryl)xanthine; AC1L3X7D; GTPL428; CTK8C1262; DTXSID30165151; ZINC5114737; 7673AA; ANW-66125; AKOS016004661; 8-(3,4-Dimethoxyphenyl)-3,7-dihydro-7-methyl-1,3-dipropyl-1H-purine-2,6-dione; AJ-53127; AX8236469; L001123; 1H-Purine-2,6-dione, 8-(3,4-dimethoxyphenyl)-3,7-dihydro-7-methyl-1,3-dipropyl-
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
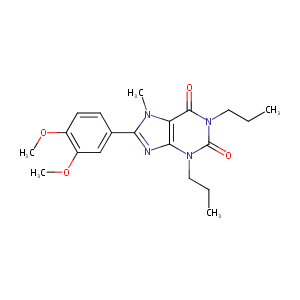 |
||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 386.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 7 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
