Details of the Drug
General Information of Drug (ID: DM8SKU1)
| Drug Name |
SR9009
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
1379686-30-2; SR-9009; Stenabolic (SR9009); SR 9009; ethyl 3-[[(4-chlorophenyl)methyl-[(5-nitrothiophen-2-yl)methyl]amino]methyl]pyrrolidine-1-carboxylate; CHEMBL1961796; 1-Pyrrolidinecarboxylic acid, 3-((((4-chlorophenyl)methyl)((5-nitro-2-thienyl)methyl)amino)methyl)-, ethyl ester; 1-pyrrolidinecarboxylic acid, 3-[[[(4-chlorophenyl)methyl][(5-nitro-2-thienyl)methyl]amino]methyl]-, ethyl ester; GTPL8901; EX-A726; BCP16215; BDBM50366238; MFCD29472236; s8692; AKOS027470307; CCG-269102; CS-4669; DB14013; SB19006; NCGC00384202-01; AC-30219; AK547297; AS-55859; HY-16989; J-690150; Q15410184; N'-[(1E)-1-(5-Chloro-2-hydroxyphenyl)ethylidene]-3-(4-morpholinylsulfonyl)benzohydrazide; ethyl 3-(((4-chlorobenzyl)((5-nitrothiophen-2-yl)methyl)amino)methyl)pyrrolidine-1-carboxylate; ethyl 3-[[(4-chlorophenyl)methyl-[(5-nitro-2-thienyl)methyl]amino]methyl]pyrrolidine-1-carboxylate; Ethyl 3-[[[(4-chlorophenyl)methyl][(5-nitro-2-thienyl)methyl]amino]methyl]-1-pyrrolidinecarboxylate
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
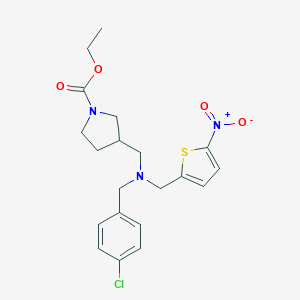 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 437.9 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.4 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 8 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
