Details of the Drug
General Information of Drug (ID: DM9AWSO)
| Drug Name |
Telapristone
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Telapristone acetate; Proellex; 198414-31-2; UNII-1K9EYK92PQ; CDB-4124; 1K9EYK92PQ; Telapristone acetate [USAN]; Telapristone acetate (USAN); CCRIS 9331; CDB 4124; SCHEMBL374762; CHEMBL2105694; DTXSID60173587; RU-44675; 17alpha-Acetoxy-11beta-(4-(dimethylamino)phenyl)-21-methoxy-19-norpregna-4,9-dien-3,20-dione; D09972; 19-Norpregna-4,9-diene-3,20-dione, 17-(acetyloxy)-11-(4-(dimethylamino)phenyl)-21-methoxy-, (11beta)-; A-Acetoxy-21-methoxy-11
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
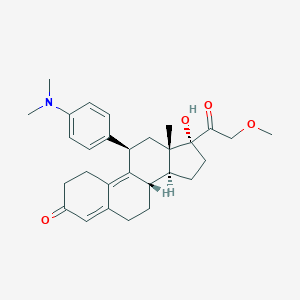 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 463.6 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.8 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||
| ADMET Property | |||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Endometriosis | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | GA10 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
