Details of the Drug
General Information of Drug (ID: DM9GDP5)
| Drug Name |
JNJ-10198409
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
JNJ-10198409; 627518-40-5; PDGFR Tyrosine Kinase Inhibitor IV; PDGF Receptor Tyrosine Kinase Inhibitor IV; CHEMBL120077; N-(3-fluorophenyl)-6,7-dimethoxy-1,4-dihydroindeno[1,2-c]pyrazol-3-amine; 3-Fluoro-N-(6,7-dimethoxy-2,4-dihydroindeno[1,2-c]pyrazol-3-yl)phenylamine; ZDNURMVOKAERHZ-UHFFFAOYSA-N; SCHEMBL3088170; GTPL6020; CTK8E8807; jnj10198409; DTXSID70430890; MolPort-009-019-131; HMS3650O03; HMS3229I11; ZINC13677843; BDBM50179207; AKOS030525202; RWJ 540973; CCG-206773; NCGC00343986-02; RT-014973; KB-274227; SR-01000946814
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
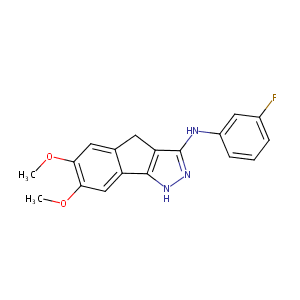 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 325.3 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.8 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
