Details of the Drug
General Information of Drug (ID: DM9IODG)
| Drug Name |
H-Phe-NH2
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
5241-58-7; Phenylalanine amide; (S)-2-amino-3-phenylpropanamide; L-Phenylalanine amide; phenylalaninamide; (2S)-2-amino-3-phenylpropanamide; Phe-NH2; L-Phe-NH2; Phenh2; UNII-PV9T9B2S11; CHEMBL350320; PV9T9B2S11; CHEBI:21371; AK-46083; phenylalanylamide; L-phenylalanineamide; Benzenepropanamide, alpha-amino-, (alphaS)-; DL-Phenylalanine amide; 2-Amino-3-phenyl-propionamide; phenylalanine imine; (S)-phenylalaninamide; Benzenepropanamide, alpha-amino-, (S)-; H-L-Phe-NH2; AC1L9IEO; SCHEMBL244302
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
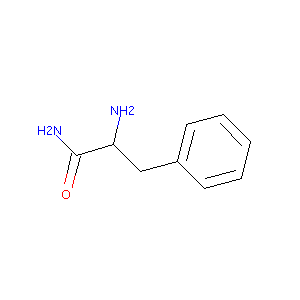 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 164.2 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 0.1 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||
