Details of the Drug
General Information of Drug (ID: DM9J35R)
| Drug Name |
GNF-PF-2094
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
GNF-Pf-2094; CHEMBL578928; AC1LF3VX; ChemDiv3_003176; MLS000555549; REGID_for_CID_697226; ZINC73452; MolPort-002-569-783; HMS2595H06; HMS3382F08; HMS1482A08; DNDI1388215; CCG-20329; BDBM50304834; STK839621; AKOS005624964; MCULE-1058130285; IDI1_021086; SMR000147266; SR-01000110418; SR-01000110418-1; BRD-K84805375-001-01-1; N2-benzyl-N4-cyclohexyl-6-methylpyrimidine-2,4-diamine; 2-N-benzyl-4-N-cyclohexyl-6-methylpyrimidine-2,4-diamine; N*2*-Benzyl-N*4*-cyclohexyl-6-methyl-pyrimidine-2,4-diamine
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
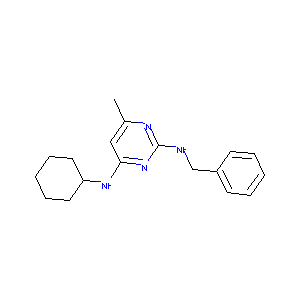 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 296.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.5 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
