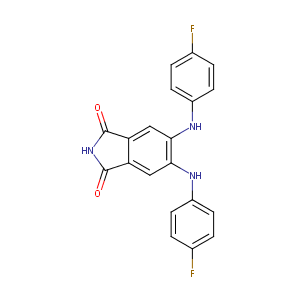
| Synonyms |
145915-60-2; CGP 53353; CGP-53353; DAPH 2; DAPH-7; CGP53353; PKCbetaII/EGFR Inhibitor; 5,6-BIS[(4-FLUOROPHENYL)AMINO]-1H-ISOINDOLE-1,3(2H)-DIONE; CHEMBL7939; CGP 53 353; 4,5-bis(4-Fluoroanilino)-phthalimide; CG53353; 5,6-Bis((4-fluorophenyl)amino)isoindoline-1,3-dione; 5,6-Bis[(4-fluorophenyl)amino]-2H-isoindole-1,3-dione; 4,5-Bis(4-fluoroanilino)phthalimide; 4,5-Bis[4-fluoroanilino]phthalimide; PKCbII/EGFR Inhibitor; AC1O8KXF; 5,6-bis(4-fluoroanilino)isoindole-1,3-dione; MolMap_000016; DAPH-2; CGP-53353, solid; SCHEMBL230492
|
| Chemical Identifiers |
- Formula
- C20H13F2N3O2
- IUPAC Name
5,6-bis(4-fluoroanilino)isoindole-1,3-dione - Canonical SMILES
-
C1=CC(=CC=C1NC2=C(C=C3C(=C2)C(=O)NC3=O)NC4=CC=C(C=C4)F)F
- InChI
-
InChI=1S/C20H13F2N3O2/c21-11-1-5-13(6-2-11)23-17-9-15-16(20(27)25-19(15)26)10-18(17)24-14-7-3-12(22)4-8-14/h1-10,23-24H,(H,25,26,27)
- InChIKey
-
RONQPWQYDRPRGG-UHFFFAOYSA-N
|