Details of the Drug
General Information of Drug (ID: DM9Y3EG)
| Drug Name |
Guadecitabine
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
UNII-2KT4YN1DP7; 929901-49-5; 2KT4YN1DP7; SGI-110 free acid; Guadecitabine [USAN:INN]; GuadecitabineSGI-110; Guadecitabine (USAN/INN); CHEMBL3544916; Guanosine, 2'-deoxy-5-azacytidylyl-(3'-5')-2'-deoxy-; ZINC43203165; AKOS027321496; AKOS030238181; DB11918; CS-3089; HY-13542; D10877; 2'-deoxy-5-azacytidylyl-(3'-5')-2'-deoxyguanosine
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Structure |
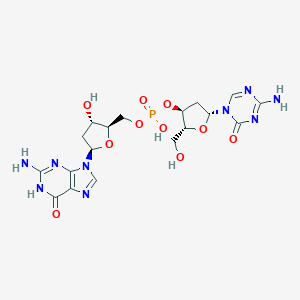 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 4 | Molecular Weight (mw) | 557.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -4.6 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 8 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 6 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 12 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Myelodysplastic syndrome | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 2A37 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
