Details of the Drug
General Information of Drug (ID: DMA3OWI)
| Drug Name |
AZD4769
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Acetic acid, ((4-phenyl-1-(4-(2-quinolinylmethoxy)phenyl)butyl)thio)-; 127481-29-2; L-674573; ((4-Phenyl-1-(4-(2-quinolinylmethoxy)phenyl)butyl)thio)acetic acid; {[4-phenyl-1-(4-(2-quinolinylmethoxy)phenyl)butyl]thio}acetic acid; L 674573; L-674,573; AC1L3YFJ; SCHEMBL9460146; CHEMBL422872; JOIXGLLMSDPZDN-UHFFFAOYSA-N; AZD-4769; 2-[[4-phenyl-1-[4-(2-quinolinylmethoxy)phenyl]butyl]thio]Acetic acid; DA-13018; FT-0734991; AZ-12096971; 2-[4-phenyl-1-[4-(quinolin-2-ylmethoxy)phenyl]butyl]sulfanylacetic
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
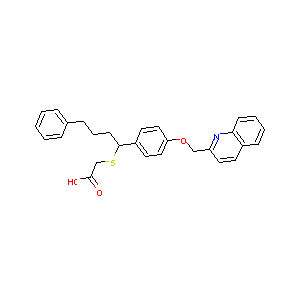 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 2 | Molecular Weight (mw) | 457.6 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 6.4 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 11 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Solid tumour/cancer | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 2A00-2F9Z | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
