Details of the Drug
General Information of Drug (ID: DMA4DMQ)
| Drug Name |
Tinostamustine
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Tinostamustine; EDO-S101; 1236199-60-2; EDO-S 101; EDO-S-101; Tinostamustine [USAN]; Tinostamustine(EDO-S101); 29DKI2H2NY; 7-(5-(bis(2-chloroethyl)amino)-1-methyl-1H-benzo[d]imidazol-2-yl)-N-hydroxyheptanamide; 1H-Benzimidazole-2-heptanamide, 5-[bis(2-chloroethyl)amino]-N-hydroxy-1-methyl-; 7-[5-[bis(2-chloroethyl)amino]-1-methylbenzimidazol-2-yl]-N-hydroxyheptanamide; Minomustine; 1H-Benzimidazole-2-heptanamide, 5-(bis(2-chloroethyl)amino)-N-hydroxy-1-methyl-; starbld0018955; UNII-29DKI2H2NY; Tinostamustine (USAN/INN); TINOSTAMUSTINE [INN]; SCHEMBL7915449; TINOSTAMUSTINE [WHO-DD]; CHEMBL3989941; BCP20331; EX-A1322; BDBM50569838; AKOS030526024; CS-6484; DB15147; SB19172; BE170657; MS-27198; HY-101780; S8769; D11182; E76854; 7-{5-[Bis(2-chloroethyl)amino]-1-methyl-1H-benzimidazol-2-yl}-N-hydroxyheptanamide
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecule
|
||||||||||||||||||||||
| Structure |
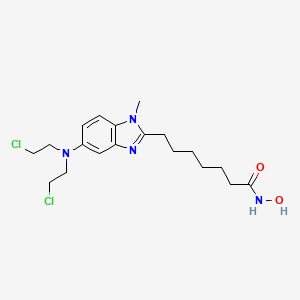 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
