Details of the Drug
General Information of Drug (ID: DMAB1RW)
| Drug Name |
L-692289
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
CHEMBL437438; L-692289; AC1MHQQ8; BDBM50285493; N-{(2R,6S,9S,11R,14aS,15S,20S,23S,25aS)-20-[(1R)-3-Amino-1-hydroxy-3-oxopropyl]-2,11,15-trihydroxy-6-[(1R)-1-hydroxyethyl]-23-[(1R)-1-hydroxy-2-(4-hydroxyphenyl)ethyl]-5,8,14,19,22,25-hexaoxotetracosahydro-1H-dipyrrolo[2,1-c:2',1'-l][1,4,7,10,13,16]hexaazacyclohenicosin-9-yl}-10,12-dimethyltetradecanamide
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Structure |
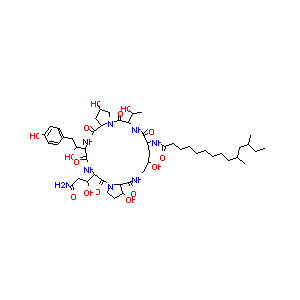 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 4 | Molecular Weight (mw) | 1033.2 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.1 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 20 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 13 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 15 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
