Details of the Drug
General Information of Drug (ID: DMABXOZ)
| Drug Name |
Hyaluronic acid
|
|||||
|---|---|---|---|---|---|---|
| Synonyms |
Hyaluronic Acid [BAN]; Hylan G-F 20; Hyvisc; Luronit; Mucoitin; Amo Vitrax; Amvisc; Biolon; Etamucine; Sepracoat; Sofast; Synvisc; C33H54N2O23; EINECS 232-678-0; HSDB 7240; Hyaluronate Sodium (hyaluronic acid)
|
|||||
| Affected Organisms |
Humans and other mammals
|
|||||
| ATC Code |
|
|||||
| Structure |
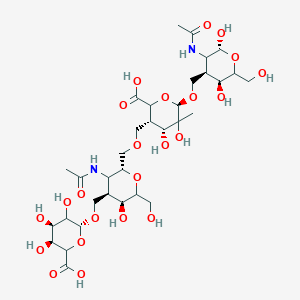 |
|||||
| 3D MOL is unavailable | 2D MOL | |||||
| #Ro5 Violations (Lipinski): 5 | Molecular Weight (mw) | 846.8 | ||||
| Logarithm of the Partition Coefficient (xlogp) | -6.8 | |||||
| Rotatable Bond Count (rotbonds) | 16 | |||||
| Hydrogen Bond Donor Count (hbonddonor) | 14 | |||||
| Hydrogen Bond Acceptor Count (hbondacc) | 23 | |||||
| ADMET Property | ||||||
| Chemical Identifiers |
|
|||||
| Cross-matching ID | ||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | |||||
Molecular Interaction Atlas of This Drug
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
