Details of the Drug
General Information of Drug (ID: DMADEHZ)
| Drug Name |
LY2880070
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
1375637-35-6; SCHEMBL15124196; BCP32047; EX-A3339; ZINC199531075; LY 2880070 pound>>LY-2880070; (R)-[5-(2-Methoxy-6-methyl-pyridin-3-yl)-2H-pyrazol-3-yl]-[6-(piperidin-3-yloxy) -pyrazin-2-yl]-amine; (R)-[5-(2-methoxy-6-methyl-pyridin-3-yl)-2H-pyrazol-3-yl]-[6-(piperidin-3-yloxy)-pyrazin-2-yl]-amine; N-[5-(2-methoxy-6-methylpyridin-3-yl)-1H-pyrazol-3-yl]-6-[(3R)-piperidin-3-yl]oxypyrazin-2-amine
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
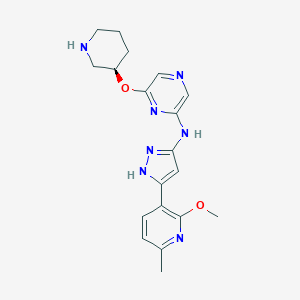 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 381.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 6 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 8 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Solid tumour/cancer | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 2A00-2F9Z | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
