Details of the Drug
General Information of Drug (ID: DMAQX4K)
| Drug Name |
(S)-tert-butyl 1-oxohexan-2-ylcarbamate
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
CHEMBL96875; ((S)-1-Formyl-pentyl)-carbamic acid tert-butyl ester; BML-244; (S)-tert-butyl 1-oxohexan-2-ylcarbamate; Carbamic acid, [(1S)-1-formylpentyl]-, 1,1-dimethylethyl ester; SCHEMBL3285479; CTK0G6629; OBMGXPJNZKYOQY-VIFPVBQESA-N; ZINC13588585; BDBM50137790; AKOS030572335; tert-butyl(1S)-1-formylpentylcarbamate; CCG-207873; 2(S)-(tert-Butoxycarbonylamino)hexanal; tert-butyl (1S)-1-formylpentylcarbamate; (2S)-2-(tert-Butoxycarbonylamino)hexanal; (S)-2-(tert-butoxycarbonylamino)-5-methylpentanal
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
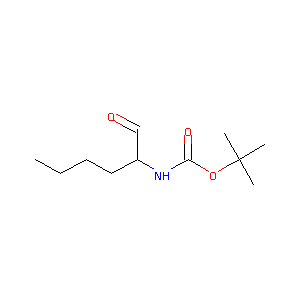 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 215.29 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.3 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 7 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
