Details of the Drug
General Information of Drug (ID: DMAS1RB)
| Drug Name |
MK-8776
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
MK-8776 Racemate; MK-8776 S-isomer; SCH900776 (Racemate); CHEMBL1643208; 6-bromo-3-(1-methyl-1H-pyrazol-4-yl)-5-(piperidin-3-yl)pyrazolo[1,5-a]pyrimidin-7-amine; 6-bromo-3-(1-methylpyrazol-4-yl)-5-piperidin-3-ylpyrazolo[1,5-a]pyrimidin-7-amine; 891495-88-8; SCH900776 S-isomer; GTPL7943; SCHEMBL10380123; CHEBI:131165; HMS3656J19; 6-BROMO-3-(1-METHYLPYRAZOL-4-YL)-5-[(3R)-PIPERIDIN-3-YL]PYRAZOLO[1,5-A]PYRIMIDIN-7-AMINE; BCP02883; BDBM50334854; NSC764659; AKOS026750519; MK 8776; NSC-764659; NCGC00387868-02; DA-40779; BCP0726000317; FT-0700501; FT-0771683; J3.517.083I; Q27086899; SCH 900776 (CAS:891494-64-7); 6-bromo-3-(1-methylpyrazol-4-yl)-5-[(3S)-piperidin-3-yl]pyrazolo[1,5-a]pyrimidin-7-amine; 6-bromo-3-(1-methylpyrazol-4-yl)-5-piperidin-3-ylpyrazolo[5,1-b]pyrimidin-7-amine
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
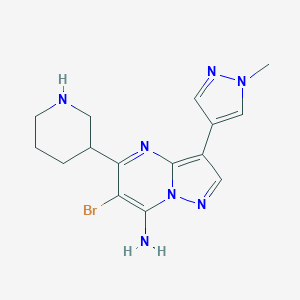 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 376.25 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 0.8 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Hodgkin lymphoma | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 2B30 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
