Details of the Drug
General Information of Drug (ID: DMAYO46)
| Drug Name |
AEZS-108
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Zoptarelin doxorubicin; AEZS-108; AN-152; UNII-27844X2J29; ZEN-008; 27844X2J29; Zoptrex; Zoptarelin doxorubicin [INN]; Zoptarelin doxorubicin [USAN]; AEZS 108; CHEMBL3544954; AN 152; Luteinizing hormone-releasing factor (pig), 6-(N6-(5-(2-(4-((3-amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyranosyl)oxy)-1,2,3,4,6,11-hexahydro-2,5,12-trihydroxy-7-methoxy-6,11-dioxo-2-naphthacenyl)-2-oxoethoxy)-1,5-dioxopentyl)-D-lysine)-, (2S-cis)-
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
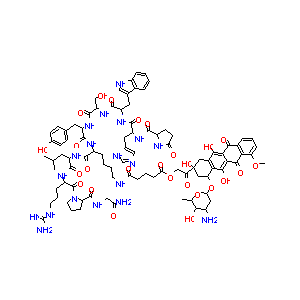 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 4 | Molecular Weight (mw) | 1893 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -0.4 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 48 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 23 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 29 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
