Details of the Drug
General Information of Drug (ID: DMAYWTI)
| Drug Name |
Boc-Tyr(SO3H)-Nle-Gly-Trp-Nle-Asp-Phe-NH2
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
CHEMBL384035; Cholecystokinin (27-33), tert-butyloxycarbonyl-nle(28,31)-; Boc-Tyr(SO3H)-Nle-Gly-Trp-Nle-Asp-Phe-NH2; 98640-66-5; Boc-28,31-nle-cck-7; BDNL; Boc-tyr(SO3H)nle-gly-trp-nle-asp-phenh2; t-Boc(nle(28,31))-cck (27-33); AC1MJ67S; DTXSID90243747; tert-Butyloxycarbonyl-28,31-nle-cholecystokinin (27-33); Cholecystokinin (27-33), tert-butyloxycarbonylnorleucyl(28,31)-; BDBM50016425; tert-Butoxycarbonyltyrosyl(sulfo)-norleucyl-glycyl-tryptophyl-norleucyl-aspartyl-phenylalaninamide
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Structure |
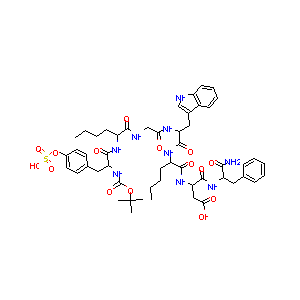 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 4 | Molecular Weight (mw) | 1092.2 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 32 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 11 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 15 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
