Details of the Drug
General Information of Drug (ID: DMB2463)
| Drug Name |
ACET
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
CHEMBL373429; 3-({3-[(2s)-2-Amino-2-Carboxyethyl]-5-Methyl-2,6-Dioxo-3,6-Dihydropyrimidin-1(2h)-Yl}methyl)-5-Phenylthiophene-2-Carboxylic Acid; (S)-1-(2-amino-2-carboxyethyl)-3-(2-carboxy-5-phenylthiophene-3-yl-methyl)-5-methylpyrimidine-2,4-dione; GTPL4123; MolPort-023-276-559; ZINC35324127; BDBM50207591; AKOS024457262; NCGC00378702-01; UBE; 3-({3-[(2S)-2-amino-2-carboxyethyl]-5-methyl-2,6-dioxo-1,2,3,6-tetrahydropyrimidin-1-yl}methyl)-5-phenylthiophene-2-carboxylic acid
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
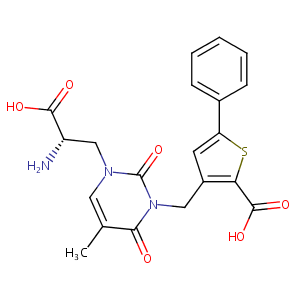 |
||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 429.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -1.1 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 7 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 8 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
