Details of the Drug
General Information of Drug (ID: DMB7WT1)
| Drug Name |
L-697,661
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
L-697661; L-697,661; 135525-78-9; L 697661; 3-(((4,7-Dichloro-1,3-benzoxazol-2-yl)methyl)amino)-5-ethyl-6-methylpyridin-2(1H)-one; CHEMBL268871; UNII-4660N666EZ; 4660N666EZ; 3-[(4,7-dichloro-1,3-benzoxazol-2-yl)methylamino]-5-ethyl-6-methyl-1H-pyridin-2-one; 2(1H)-Pyridinone, 3-(((4,7-dichloro-2-benzoxazolyl)methyl)amino)-5-ethyl-6-methyl-; ACMC-20mvsm; SCHEMBL599639; BDBM1317; AC1Q69B2; AC1L22E2; CTK0H5173; DTXSID10159457; AKOS030539285; LS-186956; LS-187602; L697,661; L 697,661
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
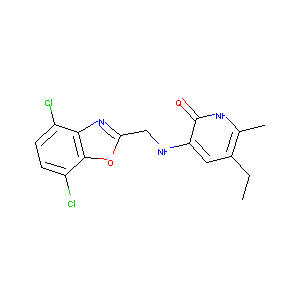 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 352.2 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.7 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
