Details of the Drug
General Information of Drug (ID: DMBG7X1)
| Drug Name |
Kaempferol-3-O-methyl ether
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Isokaempferide; 1592-70-7; 3-Methylkaempferol; 3-O-Methylkaempferol; Kaempferol 3-methyl ether; kaempferol-3-O-methyl ether; CHEBI:1579; CHEMBL165064; 4H-1-BENZOPYRAN-4-ONE, 5,7-DIHYDROXY-2-(4-HYDROXYPHENYL)-3-METHOXY-; 3-Methylkempferol; AC1NQXOY; MLS000877008; SCHEMBL4637442; SCHEMBL1628023; MEGxp0_000171; 3-MK; cid_5280862; ACon1_001687; DTXSID90166609; MolPort-001-740-349; HMS2271C11; ZINC5732268; BDBM50240899; LMPK12112688; AKOS032949069
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
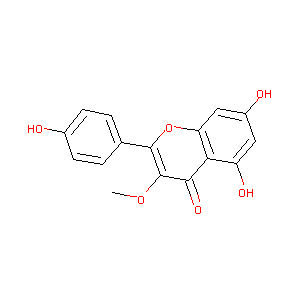 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 300.26 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.2 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
