Details of the Drug
General Information of Drug (ID: DMBQ5OL)
| Drug Name |
VIP-152
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Enitociclib; 1255AT22ZJ; UNII-1255AT22ZJ; VIP152; CHEMBL4762845; VIP-152; BAY-1251152; 1610408-97-3; 2-Pyridinamine, 5-fluoro-4-(4-fluoro-2-methoxyphenyl)-N-(4-(((S(S))-S-methylsulfonimidoyl)methyl)-2-pyridinyl)-; 2-Pyridinamine, 5-fluoro-4-(4-fluoro-2-methoxyphenyl)-N-[4-[[[S(S)]-S-methylsulfonimidoyl]methyl]-2-pyridinyl]-; ENITOCICLIB [INN]; ENITOCICLIB [USAN]; GTPL11686; GLXC-26844; BDBM50556034; BAY 1251152; csc(S)-[(2-{[5-fluoro-4-(4-fluoro-2-methoxyphenyl)pyridin-2-yl]amino}pyridin-4-yl)methyl](imino)(methyl)-l6-sulfanone
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Drug Type |
Small molecule
|
||||||||||||||||||||||||||
| Structure |
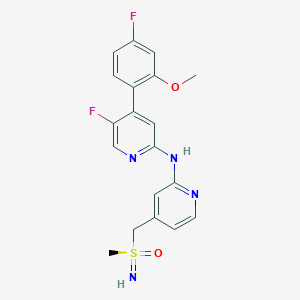 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Chronic lymphocytic leukaemia | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 2A82.0 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
