Details of the Drug
General Information of Drug (ID: DMBZ2MF)
| Drug Name |
THZ531
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
1702809-17-3; THZ-531; (R,E)-N-(4-(3-((5-chloro-4-(1H-indol-3-yl)pyrimidin-2-yl)amino)piperidine-1-carbonyl)phenyl)-4-(dimethylamino)but-2-enamide; CHEMBL4163879; THZ531 HCl; THZ-531 HCl; SCHEMBL16655248; SCHEMBL16655252; CHEBI:143122; THZ531; THZ 531; (E)-N-[4-[(3R)-3-[[5-chloro-4-(1H-indol-3-yl)pyrimidin-2-yl]amino]piperidine-1-carbonyl]phenyl]-4-(dimethylamino)but-2-enamide; AMY16834; BCP28996; EX-A1532; BDBM50528813; NSC821656; s6595; NSC-821656; SB18810; AC-31604; BS-16034; HY-103618; CS-0015451; J3.623.785F; CN(C)CC=CC(=O)Nc1ccc(cc1)C(=O)N1CCC[C@H](C1)Nc1ncc(Cl)c(n1)-c1c[nH]c2ccccc12; (2E)-N-(4-{[(3R)-3-{[5-chloro-4-(1H-indol-3-yl)pyrimidin-2-yl]amino}piperidin-1-yl]carbonyl}phenyl)-4-(dimethylamino)but-2-enamide
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
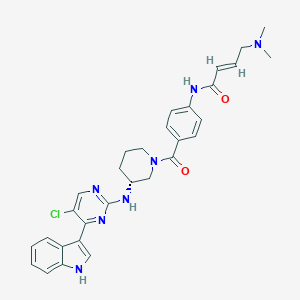 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 558.1 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.1 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 8 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
