Details of the Drug
General Information of Drug (ID: DMC0JN8)
| Drug Name |
GYKI-53655
|
|||||
|---|---|---|---|---|---|---|
| Synonyms |
GYKI-53655; Gyki 53655; GYKI53655; 1-(4-AMINOPHENYL)-3-METHYLCARBAMOYL-4-METHYL-7,8-METHYLENEDIOXY-3,4-DIHYDRO-5H-2,3-BENZODIAZEPINE; 143692-18-6; CHEMBL267450; LY 300168; SMGACXZFVXKEAX-UHFFFAOYSA-N; AC1L2QV0; GYKI-53655 free base; MLS006010331; SCHEMBL351325; GTPL4209; DTXSID30276296; MolPort-006-418-196; BCP27661; BDBM50048386; ABP000447; NCGC00263113-03; NCGC00263113-01; SMR004701394; LY300168; LY-300168; FT-0765288; LY 300168(GYKI 53655); LY-300168, (+/-)-; 7H-1,3-Dioxolo(4,5-H)(2,3)benzodiazepine-7-carboxamide, 5-(4-aminopheny
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Structure |
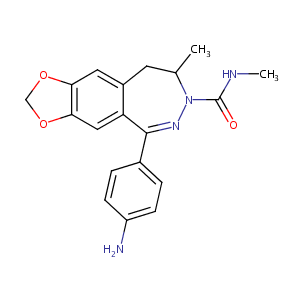 |
|||||
| 3D MOL | 2D MOL | |||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 352.4 | ||||
| Logarithm of the Partition Coefficient (xlogp) | 2.6 | |||||
| Rotatable Bond Count (rotbonds) | 1 | |||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | |||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | |||||
| Chemical Identifiers |
|
|||||
| Cross-matching ID | ||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | |||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
|
||||||||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
