Details of the Drug
General Information of Drug (ID: DMC35T1)
| Drug Name |
BN50727
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Setipafant; Setipafant [INN]; UNII-UFN2Q54HS6; UFN2Q54HS6; 132418-35-0; BN 50727; 6-(o-Chlorophenyl)-7,10-dihydro-1-methyl-4H-pyrido(4',3':4,5)thieno(3,2-f)-s-triazolo(4,3-a)(1,4)diazepine-9(8H)-carbox-p-anisidide; 4H-Pyrido(4',3':4,5)thieno(3,2-f)(1,2,4)triazolo(4,3-a)(1,4)diazepine-9(8H)-carboxamide, 7,10-dihydro-6-(2-chlorophenyl)-N-(4-methoxyphenyl)-1-methyl-; BN50727; AC1L24CK; AC1Q3P7H; SCHEMBL2110462; CHEMBL2107078; DTXSID90157552; ZINC1481922; CS-6678; BN-50727; LS-134347; HY-101675
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
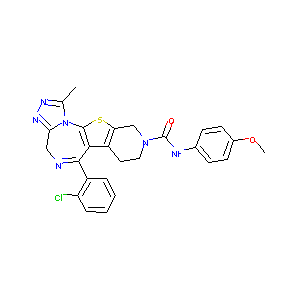 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 519 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Inflammatory bowel disease | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | DD72 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
