Details of the Drug
General Information of Drug (ID: DMCFLRH)
| Drug Name |
Cibinetide
|
||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
UNII-9W5677JKDA; 9W5677JKDA; 1208243-50-8; Cibinetide [USAN]; Pglu-glu-gln-leu-glu-arg-ala-leu-asn-ser-ser; Pyr-glu-gln-leu-glu-arg-ala-leu-asn-ser-ser-OH; GTPL9677; CHEMBL3545305; DB13006; L-Serine, 5-oxo-L-prolyl-L-alpha-glutamyl-L-glutaminyl-L-leucyl-L-alpha-glutamyl-L-arginyl-L-alanyl-L-leucyl-L-asparaginyl-L-seryl-; L-Pyroglutamyl-L-glutamyl-L-glutaminyl-L-leucyl-L-glutamyl-L-arginyl-L-alanyl-L-leucyl-L-asparaginyl-L-seryl-L-serine; L-Pyroglutamyl-L-glutamyl-L-glutam
|
||||||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||||||
| Structure |
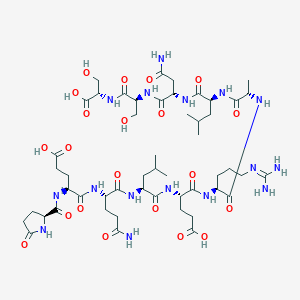 |
||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 5 | Molecular Weight (mw) | 1257.3 | |||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -7.7 | ||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 42 | ||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 20 | ||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 22 | ||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Depression | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 6A70-6A7Z | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
