Details of the Drug
General Information of Drug (ID: DMCU6XV)
| Drug Name |
Acid blue 25
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Acid Blue 25; 6408-78-2; C.I. ACID BLUE 25; UNII-S2E15W6FSN; S2E15W6FSN; W-110136; CHEMBL256057; 2-Anthracenesulfonic acid, 1-amino-9,10-dihydro-9,10-dioxo-4-(phenylamino)-, sodium salt (1:1); EINECS 229-068-1; EC 229-068-1; 2-Anthracenesulfonic acid, 1-amino-9,10-dihydro-9,10-dioxo-4-(phenylamino)-, monosodium salt; C20H13N2NaO5S; SCHEMBL790822; DTXSID2044711; CTK8F7553; Sodium 1-amino-9,10-dioxo-4-phenylaminoanthracene-2-sulphonate; MFCD00001214; Acid Blue 25, Dye content 45 %; AKOS015894441; AN-19165; Q394; I04-8965; sodium 1
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
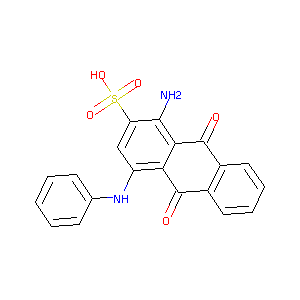 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 |
Molecular Weight | 416.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient | Not Available | ||||||||||||||||||||||
| Rotatable Bond Count | 3 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count | 7 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
