Details of the Drug
General Information of Drug (ID: DMCZSP3)
| Drug Name |
5-methoxycarbonylamino-N-acetyltryptamine
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
5-Methoxycarbonylamino-N-acetyltryptamine; GR 135531; 190277-13-5; 5-MCA-NAT; 5MCA-NAT; CHEMBL504585; methyl 3-(2-acetamidoethyl)-1H-indol-5-ylcarbamate; methyl N-[3-(2-acetamidoethyl)-1H-indol-5-yl]carbamate; Methyl {3-[2-(Acetylamino)ethyl]-1h-Indol-5-Yl}carbamate; Methyl [3-[2-(acetylamino)ethyl]-1H-indol-5-yl]carbamate; Tocris-0896; AC1N7T0H; GTPL3393; SCHEMBL4655625; CTK6J2921; CHEBI:93457; DTXSID70401560; MPZVHKLZCUEJFO-UHFFFAOYSA-N; MolPort-003-848-587; HMS3266P16; ZINC2567732; BN0231; BDBM50260394; PDSP2_001790
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
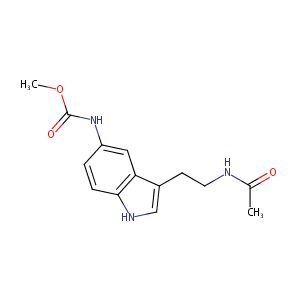 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 275.3 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 0.4 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
References
