Details of the Drug
General Information of Drug (ID: DMD0K61)
| Drug Name |
PT-102
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
HI-275; CHEMBL317356; Thiourea, N-(5-bromo-2-pyridinyl)-N'-(2-phenylethyl)-; 149487-00-3; PETT Analog 52; PT-102; AC1M6SPL; MLS000517095; BDBM1885; SCHEMBL7624466; cid_2429552; MolPort-002-358-086; HMS2674B12; STK928015; ZINC12487116; AKOS001055750; MCULE-2726995695; NCGC00246073-01; SMR000343246; 1-(5-bromopyridin-2-yl)-3-phenethylthiourea; 1-(5-bromo-2-pyridyl)-3-phenethyl-thiourea; N-(2-Phenethyl)-N -(2-(5-bromopyridyl))thiourea; 1-(5-bromopyridin-2-yl)-3-(2-phenylethyl)thiourea
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
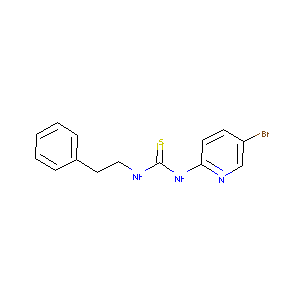 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 336.25 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.4 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
