Details of the Drug
General Information of Drug (ID: DMD3C21)
| Drug Name |
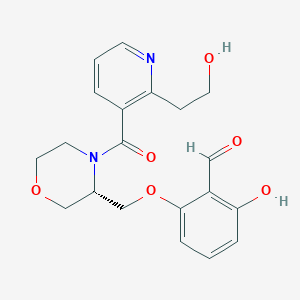
GBT021601
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Osivelotor; GBT-601; GBT021601; GBT-021601; 2417955-18-9; osivelotor [INN]; 2-hydroxy-6-({(3S)-4-[2-(2-hydroxyethyl)pyridine-3-carbonyl]morpholin-3-yl}methoxy)benzaldehyde; 2-hydroxy-6-[[(3S)-4-[2-(2-hydroxyethyl)pyridine-3-carbonyl]morpholin-3-yl]methoxy]benzaldehyde; SCHEMBL21957946; GTPL12856; UK749B4S16; example 8 [US20210047309]; HY-148788; CS-0641066; 2-hydroxy-6-{[(3S)-4-{[2-(2-hydroxyethyl)pyridin-3- yl]carbonyl}morpholin-3-yl]methoxy}benzaldehyde
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecule
|
||||||||||||||||||||||
| Structure |
 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
