Details of the Drug
General Information of Drug (ID: DMD5FIU)
| Drug Name |
Clorobiocin
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Clorobiocin; Chlorobiocin; Antibiotic RP 18,631; NSC 227186; RP 18631; 18631 RP; 39868-96-7; CHEMBL303984; 1H-Pyrrole-2-carboxylic acid, 5-methyl-, 3'-ester with N-(8-chloro-7-((6-deoxy-5-C-methyl-4-O-methyl-alpha-L-lyxo-hexopyranosyl)oxy)-4-hydroxy-2-oxo-2H-1-benzopyran-3-yl)-4-hydroxy-3-(3-methyl-2-butenyl)benzamide; [(3R,4S,5R,6S)-6-[8-chloro-4-hydroxy-3-[[4-hydroxy-3-(3-methylbut-2-enyl)benzoyl]amino]-2-oxo-chromen-7-yl]oxy-5-hydroxy-3-methoxy-2,2-dimethyl-tetrahydropyran-4-yl] 5-methyl-1H-pyrrole-2-carboxylate
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Affected Organisms |
Enteric bacteria and other eubacteria
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
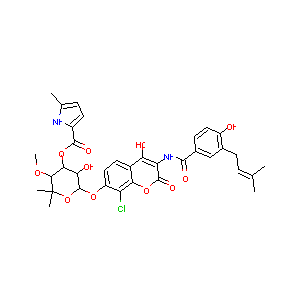 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 3 | Molecular Weight (mw) | 697.1 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 5.3 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 10 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 5 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 11 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
