Details of the Drug
General Information of Drug (ID: DMD6N8V)
| Drug Name |
N-(1-Adamantyl)-N'-(4-Guanidinobenzyl)Urea
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
n-(1-adamantyl)-n'-(4-guanidinobenzyl)urea; AGB; 1ejn; AC1L1HTN; SCHEMBL4328331; WX293T; SCHEMBL14524522; CTK7G4253; BDBM16176; WX-293T; DB03782; 3-adamantan-1-yl-1-[(4-carbamimidamidophenyl)methyl]urea; 3-(1-adamantyl)-1-[(4-carbamimidamidophenyl)methyl]urea; 3-(adamantan-1-yl)-1-[(4-carbamimidamidophenyl)methyl]urea; 1-(1-adamantyl)-3-[[4-(diaminomethylideneamino)phenyl]methyl]urea; 1-{4-[(diaminomethylidene)amino]benzyl}-3-tricyclo[3.3.1.13,7]dec-1-ylurea
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
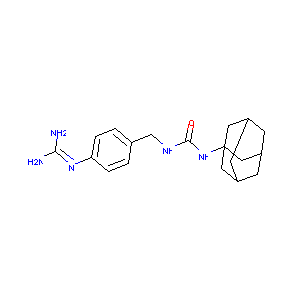 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 341.5 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
