Details of the Drug
General Information of Drug (ID: DMD9Y5T)
| Drug Name |
AZD7648
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
AZD-7648; 2230820-11-6; UNII-97A09L5JCK; 97A09L5JCK; 7-methyl-2-((7-methyl-[1,2,4]triazolo[1,5-a]pyridin-6-yl)amino)-9-(tetrahydro-2H-pyran-4-yl)-7,9-dihydro-8H-purin-8-one; 7-Methyl-2-((7-methyl-[1,2,4]triazolo[1,5-a]pyridin-6-yl)amino)-9-(tetrahydro-2H-pyran-4-yl)-7H-purin-8(9H)-one; 7-methyl-2-[(7-methyl-[1,2,4]triazolo[1,5-a]pyridin-6-yl)amino]-9-(oxan-4-yl)purin-8-one; CHEMBL4439259; SCHEMBL20299477; GTPL10601; US10407446, Example 3; BDBM413450; BCP30676; EX-A2988; AZD 7648;AZD7648; MFCD32062688; NSC817043; s8843; ZB1555; NSC-817043; SB23233; compound 16 [PMID: 31851518]; AC-31586; BS-16016; HY-111783; CS-0091859; CN1C(=O)N(C2CCOCC2)C2=NC(NC3=CN4N=CN=C4C=C3C)=NC=C12; 7-Methyl-2-((7-methyl(1,2,4)triazolo(1,5- a)pyridin-6-yl)amino)-9-(tetrahydro-2H-pyran- 4-yl)-7,9-dihydro-8H-purin-8-one; 8H-Purin-8-one, 7,9-dihydro-7-methyl-2-((7- methyl(1,2,4)triazolo(1,5-a)pyridin-6- yl)amino)-9-(tetrahydro-2H-pyran-4-yl)-
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
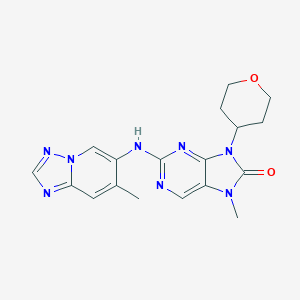 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 380.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 7 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
