Details of the Drug
General Information of Drug (ID: DMDC71B)
| Drug Name |
AcAsp-D-Glu-Leu-Glu-Cha-Cys
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
AcAsp-D-Glu-Leu-Glu-Cha-Cys; CHEMBL60967; AC1LAOO4; BDBM50096410; AcAsp-D-Glu-Leu-Glu-.beta.-Cyclohexylalanine-Cys; Ac-L-Asp-D-Glu-L-Leu-L-Glu-3-Cyclohexyl-L-Ala-L-Cys-OH; (4S)-4-[[(2S)-2-[[(2R)-2-[[(2S)-2-acetamido-4-hydroxy-4-oxobutanoyl]amino]-5-hydroxy-5-oxopentanoyl]amino]-4-methylpentanoyl]amino]-5-[[(2S)-3-cyclohexyl-1-[[(2R)-1-hydroxy-1-oxo-3-sulfanylpropan-2-yl]amino]-1-oxopropan-2-yl]amino]-5-oxopentanoic acid
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
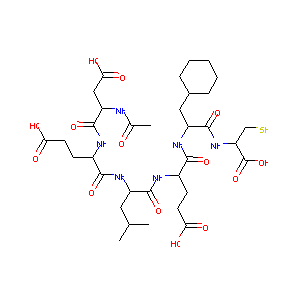 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 4 | Molecular Weight (mw) | 802.9 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 0.3 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 25 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 11 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 15 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
