Details of the Drug
General Information of Drug (ID: DMDPQV5)
| Drug Name |
BMS-599626
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
BMS-599626; 714971-09-2; AC480; AC-480; BMS 599626; (S)-Morpholin-3-ylmethyl (4-((1-(3-fluorobenzyl)-1H-indazol-5-yl)amino)-5-methylpyrrolo[2,1-f][1,2,4]triazin-6-yl)carbamate; UNII-2252724U5N; CHEMBL1645462; BMS599626; AC480 (BMS-599626); 2252724U5N; J-502499; C27H27FN8O3; AC 480; AC1480 (free base); PubChem22432; GTPL7647; SCHEMBL12065349; CTK8B6780; DTXSID60221714; SYN1142; AOB5594; MolPort-023-279-418; EX-A2296; ZINC6717782; KS-000008GE; BDBM50333373; s1056; C27H44O11; ANW-54337; AKOS015999853; CS-0407; RL05125; BCP9000428; SB21703
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Anticancer Agents
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
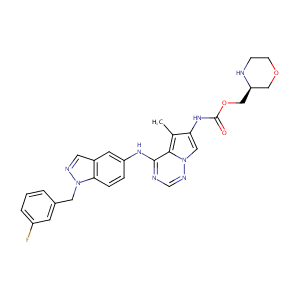 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 530.6 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.8 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 8 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 9 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Solid tumour/cancer | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 2A00-2F9Z | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
