Details of the Drug
General Information of Drug (ID: DMELJDI)
| Drug Name |
KAI-9803
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
DELCASERTIB; UNII-5G7N7E908H; 5G7N7E908H; KAI-9803; BMS-875944; Delcasertib [USAN:INN]; 949100-39-4; HY-106262; CS-0025460; L-Arginine, L-cysteinyl-L-tyrosylglycyl-L-arginyl-L-lysyl-L-lysyl-L-arginyl-L-arginyl-L- glutaminyl-L-arginyl-L-arginyl-, (1->1')-disulfide with L-cysteinyl-L-seryl-L-phenylalanyl-L- asparaginyl-L-seryl-L-tyrosyl-L-alpha-glutamyl-L-leucylglycyl-L-seryl-L-leucine; L-Cysteinyl-L-tyrosylglycyl-L-arginyl-L-lysyl-L-lysyl-L-arginyl-L-arginyl-L-glutaminyl-L- arginyl-L-arginyl-L-arginine (1->1')-d
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
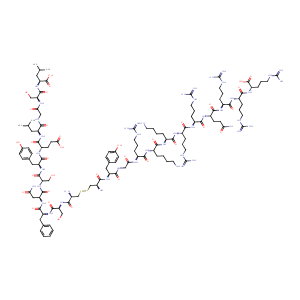 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 5 | Molecular Weight (mw) | 2880.3 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -19.7 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 108 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 53 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 46 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Acute myocardial infarction | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | BA41 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
