Details of the Drug
General Information of Drug (ID: DMFPOUC)
| Drug Name |
CGP-57380
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
CGP 57380; 522629-08-9; CGP-57380; MNK1 Inhibitor; N3-(4-fluorophenyl)-1h-pyrazolo[3,4-d]pyrimidine-3,4-diamine; CGP57380; CHEMBL1240885; C11H9FN6; 4-Amino-5-(4-fluoroanilino)-pyrazolo[3,4-d]pyrimidine; SCHEMBL987991; GTPL6010; CHEMBL576817; SCHEMBL16714452; CHEBI:92749; DTXSID50469941; MolPort-006-725-822; HMS3653G22; HMS3269P13; HMS3263L14; HMS3229K20; HMS3648M14; Tox21_501256; BDBM50130693; 2314AH; NSC741567; MFCD03861062; ZINC13816313; s7421; IN1236; BDBM50298223; AKOS024457265; CCG-206868; NSC-741567; LP01256; NCGC00162380-06
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
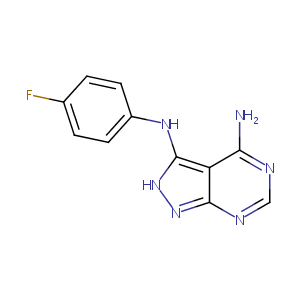 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 244.23 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.3 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
