Details of the Drug
General Information of Drug (ID: DMG4RFO)
| Drug Name |
TAK-442
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Letaxaban; TAK-442; 870262-90-1; UNII-Y3WB03966W; TAK 442; CHEMBL1095032; Y3WB03966W; 1-(1-{(2S)-3-[(6-Chloronaphthalen-2-yl)sulfonyl]-2-hydroxypropanoyl}piperidin-4-yl)tetrahydropyrimidin-2(1H)-one; 2(1H)-Pyrimidinone,1-[1-[(2S)-3-[(6-chloro-2-naphthalenyl)sulfonyl]-2-hydroxy-1-oxopropyl]-4-piperidinyl]tetrahydro-; Letaxaban [USAN:INN]; 1-[1-[(2S)-3-[(6-Chloronaphthalen-2-yl)sulfonyl]-2-hydroxypropanoyl]piperidin-4-yl]tetrahydropyrimidin-2(1H)-one; 3kl6; Letaxaban (USAN/INN); SCHEMBL766143; GEHAEMCVKDPMKO-HXUWFJFHSA-N
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
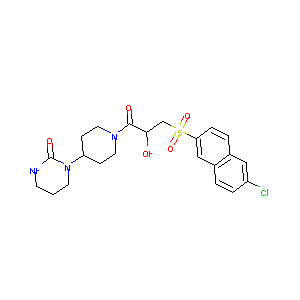 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 480 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.9 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Thrombosis | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | DB61-GB90 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
