Details of the Drug
General Information of Drug (ID: DMG7YQL)
| Drug Name |
GSK2245840
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Gepirone hydrochloride; GEPIRONE HYDROCHLORIDE; Gepirone HCl; UNII-80C9L8EP6V; Gepirone hydrochloride [USAN]; 80C9L8EP6V; 83928-66-9; CHEMBL1204187; Gepirone hydrochloride (USAN); BMY 138951; AC1Q3ELB; AC1L1IK3; SCHEMBL318838; DTXSID30232812; AOB5299; 83928-76-1 (Parent); ORG-33062; SB19633; BMY-13805-1; BMY 13805-1; 3,3-Dimethyl-1-(4-(4-(2-pyrimidinyl)-1-piperazinyl)butyl)glutarimide monohydrochloride; D04314; 4,4-dimethyl-1-[4-(4-pyrimidin-2-ylpiperazin-1-yl)butyl]piperidine-2,6-dione hydrochloride; 2,6-Piperidinedione,
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
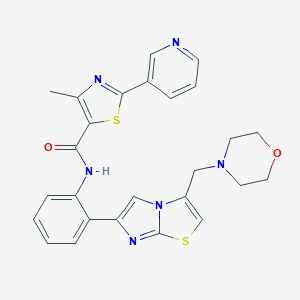 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 516.6 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.2 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 6 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 8 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Chronic obstructive pulmonary disease | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | CA22 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
