Details of the Drug
General Information of Drug (ID: DMGBLI3)
| Drug Name |
VS-6063
|
||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Defactinib hydrochloride; 1073160-26-5; Defactinib (hydrochloride); UNII-L2S469LM49; Defactinib hydrochloride [USAN]; L2S469LM49; Defactinib hydrochloride (USAN); Benzamide, N-methyl-4-[[4-[[[3-[methyl(methylsulfonyl)amino]-2-pyrazinyl]methyl]amino]-5-(trifluoromethyl)-2-pyrimidinyl]amino]-, hydrochloride; Defactinib HCl; Benzamide, N-methyl-4-((4-(((3-(methyl(methylsulfonyl)amino)-2-pyrazinyl)methyl)amino)-5-(trifluoromethyl)-2-pyrimidinyl)amino)-, hydrochloride (1:1); Benzamide, N-methyl-4-[[4-[[[3-[methyl(methylsu
|
||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||
| Structure |
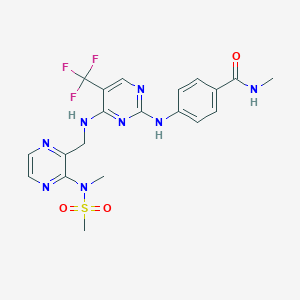 |
||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 2 | Molecular Weight (mw) | 510.5 | |||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.4 | ||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 8 | ||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 13 | ||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
