Details of the Drug
General Information of Drug (ID: DMH4HEW)
| Drug Name |
rhenium-186
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms | Rhenium-186; Rhenium Re-186; ZU7F1ET6TM; 14998-63-1; (~186~Re)Rhenium; UNII-ZU7F1ET6TM; 186Re; RHENIUM (186-RE); Rhenium, isotope of mass 186; DTXSID60933825; RHENIUM-186 [WHO-DD]; Q18882928 | ||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecule
|
||||||||||||||||||||||
| Structure |
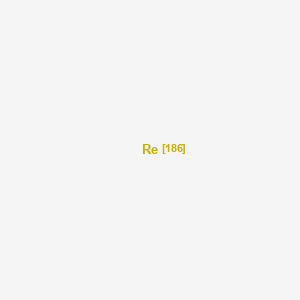 |
||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
