Details of the Drug
General Information of Drug (ID: DMH5CAQ)
| Drug Name |
4-(2-amino-1,3-thiazol-4-yl)phenol
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
4-(2-Amino-1,3-thiazol-4-yl)phenol; 57634-55-6; 4-(2-amino-4-thiazolyl)phenol; 4-(2-Amino-thiazol-4-yl)-phenol; 4-(2-aminothiazol-4-yl)phenol; CHEMBL483790; QGSJYYIRAFRPIT-UHFFFAOYSA-N; Phenol, 4-(2-amino-4-thiazolyl)-; 2-amino-4-(4-hydroxyphenyl)-thiazole; NSC405294; 3fu3; Oprea1_415030; SCHEMBL5321407; p-(2-Amino-4-thiazolyl)phenol; CTK5A7198; DTXSID30323956; MolPort-000-141-769; HMS3604B06; Phenol,4-(2-amino-4-thiazolyl)-; AC1L8637; KS-00000A4L; ZINC16951624; STK723345; SBB018017; BDBM50293592; KM0129; 5926AE; 4-(2-Aminothiazol-4-yl)phenol
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
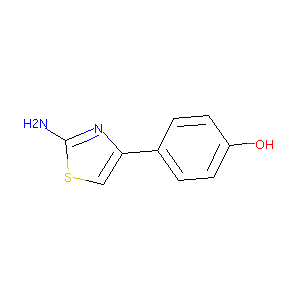 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 192.24 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.2 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
