Details of the Drug
General Information of Drug (ID: DMHA5ME)
| Drug Name |
AZD-9684
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Laudanosine; DL-Laudanosine; 1699-51-0; (+-)-Laudanosine; Laudanosine (R,S); (R,S)-Laudanosine; EINECS 216-923-9; NSC 94267; AI3-61890; 20412-65-1; NSC35045; 1-[(3,4-dimethoxyphenyl)methyl]-6,7-dimethoxy-2-methyl-1,2,3,4-tetrahydroisoquinoline; (+-)-1,2,3,4-Tetrahydro-6,7-dimethoxy-2-methyl-1-veratrylisoquinoline; 1-(3,4-dimethoxybenzyl)-6,7-dimethoxy-2-methyl-1,2,3,4-tetrahydroisoquinoline; ISOQUINOLINE, 1,2,3,4-TETRAHYDRO-6,7-DIMETHOXY-2-METHYL-1-VERATRYL-, (+-)-
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
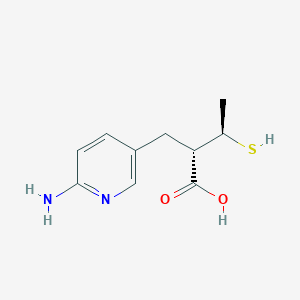 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 226.3 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Thrombosis | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | DB61-GB90 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
