Details of the Drug
General Information of Drug (ID: DMHW289)
| Drug Name |
DCC-3014
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Vimseltinib; UNII-PX9FTM69BF; PX9FTM69BF; DCC3014; 1628606-05-2; 2-(isopropylamino)-3-methyl-5-(6-methyl-5-((2-(1-methyl-1H-pyrazol-4-yl)pyridin-4-yl)oxy)pyridin-2-yl)pyrimidin-4(3H)-one; 3-methyl-5-[6-methyl-5-[2-(1-methylpyrazol-4-yl)pyridin-4-yl]oxypyridin-2-yl]-2-(propan-2-ylamino)pyrimidin-4-one; Vimseltinib [INN]; SCHEMBL16047448; GTPL11190; EX-A4700; NSC828316; NSC-828316; example 10 [WO2014145025A2]; HY-136256; CS-0121044; 3-Methyl-2-((1-methylethyl)amino)-5-(6-methyl-5-((2-(1-methyl-1H-pyrazol-4-yl)-4-pyridinyl)oxy)-2-pyridinyl)-4(3H)-pyrimidinone; 4(3H)-Pyrimidinone, 3-methyl-2-((1-methylethyl)amino)-5-(6-methyl-5-((2-(1-methyl-1H-pyrazol-4-yl)-4-pyridinyl)oxy)-2-pyridinyl)-
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
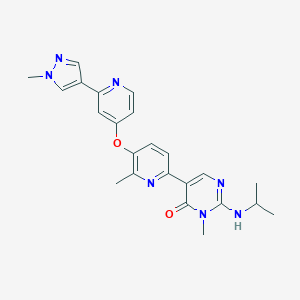 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 431.5 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.5 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 6 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
