Details of the Drug
General Information of Drug (ID: DMI7JF3)
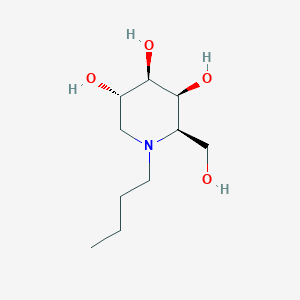
| Drug Name |
Lucerastat
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
141206-42-0; UNII-GVS3YDM418; N-Butyldeoxygalactonojirimycin; GVS3YDM418; CHEMBL1086997; N-(n-Butyl)deoxygalactonojirimycin; (2R,3S,4R,5S)-1-butyl-2-(hydroxymethyl)piperidine-3,4,5-triol; Lucerastat [INN]; N-Butyl-DGJ; NB-DGJ; N-Bu-DGJ; AC1L9UXN; N- Butyldeoxygalactonojirimycin; SCHEMBL6821044; N-Butyl-deoxy-galactonojirimycin; CDP-923; N-N-Butyl Deoxygalactonojirimycin; OGT-923; DTXSID60161601; MolPort-039-015-418; ZX-AT009021; ZINC13719785; BDBM50312528; N-Butyl-D-galacto-1-deoxynojirimycin; AKOS027384398; NCGC00181326
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Structure |
 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
