Details of the Drug
General Information of Drug (ID: DMIKJNL)
| Drug Name |
S6-nitrobenzyl mercaptopurine riboside
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
NBMPR; 38048-32-7; 4-Nitrobenzylthioinosine; NBTI; 6-(p-Nitrobenzylthio)inosine; EINECS 253-753-4; UNII-GV1L2DZM2Z; GV1L2DZM2Z; BRN 1191080; 6-((4-Nitrobenzyl)thio)-9-beta-D-ribofuranosylpurine; CHEMBL418509; (2R,3S,4R,5R)-2-(Hydroxymethyl)-5-(6-((4-nitrobenzyl)thio)-9H-purin-9-yl)tetrahydrofuran-3,4-diol; 6-(((4-Nitrophenyl)methyl)thio)-9-beta-D-ribofuranosyl-9H-purine; 9H-Purine, 6-(((4-nitrophenyl)methyl)thio)-9-beta-D-ribofuranosyl-
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
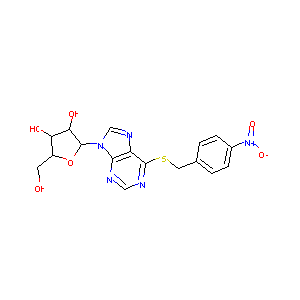 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 419.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.5 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 10 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
