Details of the Drug
General Information of Drug (ID: DMILMHZ)
| Drug Name |
BMS-564929
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
BMS-564929; 627530-84-1; UNII-9BLW27W4X7; BMS 564929; 9BLW27W4X7; C14H12ClN3O3; 2-Chloro-4-[(7r,7as)-7-Hydroxy-1,3-Dioxotetrahydro-1h-Pyrrolo[1,2-C]imidazol-2(3h)-Yl]-3-Methylbenzonitrile; 2-Chloro-3-methyl-4-[(7R,7AS)-tetrahydro-7-hydroxy-1,3-dioxo-1H-pyrrolo[1,2-c]imidazol-2(3H)-yl]benzonitrile; KEJORAMIZFOODM-PWSUYJOCSA-N; 2nw4; SCHEMBL2967577; DTXSID50432357; ZINC3938678; 3500AH; AKOS027288930; CS-1381; DB07286; BMS-564,929; NCGC00378909-01; HY-12111; 8NH; KB-75607; BMS 564929;BMS564929; W-5594; S900006120
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
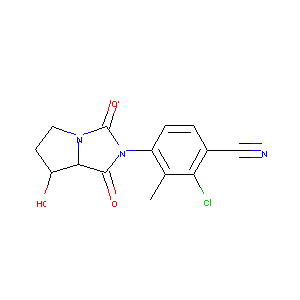 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 305.71 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.1 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
