| Drug Name |
R1507
|
| Indication |
| Disease Entry |
ICD 11 |
Status |
REF |
| Graves ophthalmopathy |
9C82.3
|
Phase 2 |
[1] |
| ------------------------------------------------------------------------------------ |
|
|
|
|
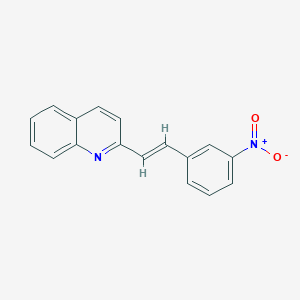
| Structure |
|
 |
|
3D MOL
|
2D MOL
|
| #Ro5 Violations (Lipinski): 0 |
Molecular Weight (mw) |
276.29 |
|
| Logarithm of the Partition Coefficient (xlogp) |
4.3 |
| Rotatable Bond Count (rotbonds) |
2 |
| Hydrogen Bond Donor Count (hbonddonor) |
0 |
| Hydrogen Bond Acceptor Count (hbondacc) |
3 |
| Chemical Identifiers |
- Formula
- C17H12N2O2
- IUPAC Name
2-[(E)-2-(3-nitrophenyl)ethenyl]quinoline - Canonical SMILES
-
C1=CC=C2C(=C1)C=CC(=N2)/C=C/C3=CC(=CC=C3)[N+](=O)[O-]
- InChI
-
InChI=1S/C17H12N2O2/c20-19(21)16-6-3-4-13(12-16)8-10-15-11-9-14-5-1-2-7-17(14)18-15/h1-12H/b10-8+
- InChIKey
-
URIXDBULDXTIHZ-CSKARUKUSA-N
|
| Cross-matching ID |
- PubChem CID
- 5376617
- CAS Number
-
- TTD ID
- D03BHM
|
|
|
|
|
|
|
|


